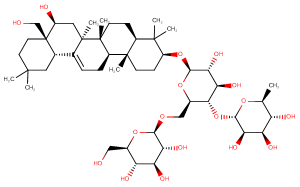
Saikosaponin F
CAS No. 62687-63-2
Saikosaponin F( —— )
Catalog No. M21524 CAS No. 62687-63-2
Saikosaponin F is a natural compound found in Bupleurum (B.) falcatum L.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 101 | Get Quote |


|
| 10MG | 147 | Get Quote |


|
| 25MG | 245 | Get Quote |


|
| 50MG | 354 | Get Quote |


|
| 100MG | 529 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameSaikosaponin F
-
NoteResearch use only, not for human use.
-
Brief DescriptionSaikosaponin F is a natural compound found in Bupleurum (B.) falcatum L.
-
DescriptionSaikosaponin F is a natural compound found in Bupleurum (B.) falcatum L.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number62687-63-2
-
Formula Weight929.1
-
Molecular FormulaC48H80O17
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (107.63 mM)
-
SMILESC[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@@H]2[C@H](O[C@H]([C@@H]([C@H]2O)O)O[C@H]3CC[C@]4([C@H](C3(C)C)CC[C@@]5([C@@H]4CC=C6[C@]5(C[C@@H]([C@@]7([C@H]6CC(CC7)(C)C)CO)O)C)C)C)CO[C@H]8[C@@H]([C@H]([C@@H]([C@H](O8)CO)O)O)O)O)O)O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Nakahara Y et al. Oleanene glycosides of the aerial parts and seeds of Bupleurum falcatum and the aerial parts of Bupleurum rotundifolium and their evaluation as anti-hepatitis agents. Chem Pharm Bull (Tokyo). 2011;59(11):1329-39.
molnova catalog



related products
-
Imeglimin
Imeglimin (EMD 387008) is an orally available antidiabetic compound that enhances mitochondrial function, enhances insulin secretion, promotes β-cell proliferation and improves pancreatic β-cell survival in mice.
-
10-O-trans-p-Feruloy...
The herbs of Hedyotis diffusa Willd.
-
Sphingosine (d17:1)
Sphingosine (d17:1) (C17 Sphingosine) is a 17-carbon sphingolipid found in human skin that can be phosphorylated by sphingomyelin kinase.Sphingosine C-17 can be used as an internal standard to perform spectroscopic analysis of sphingolipids.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


